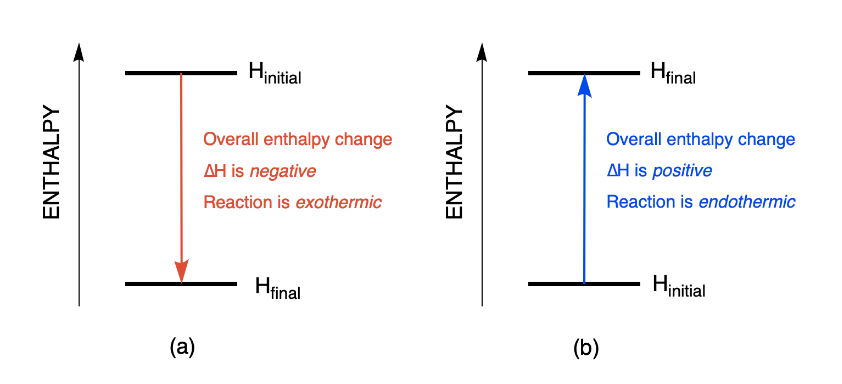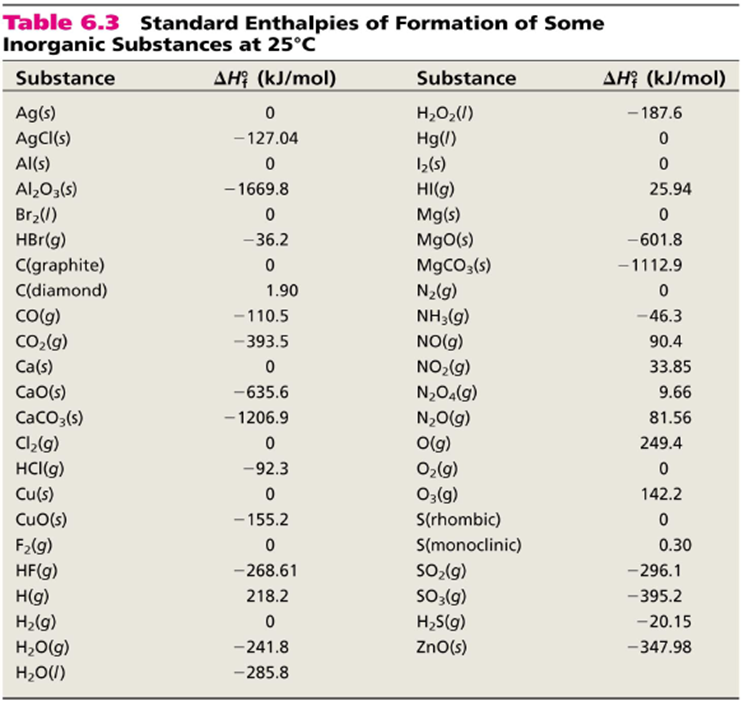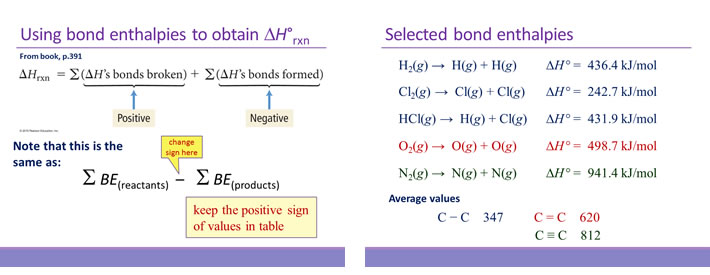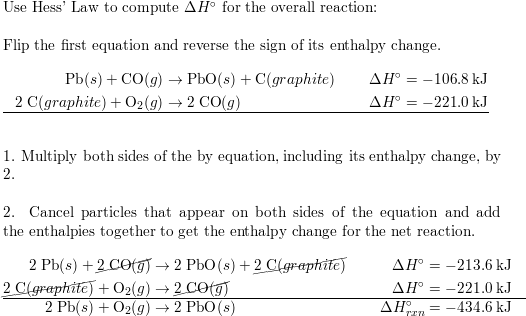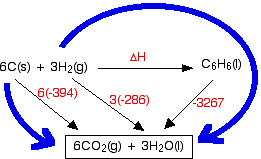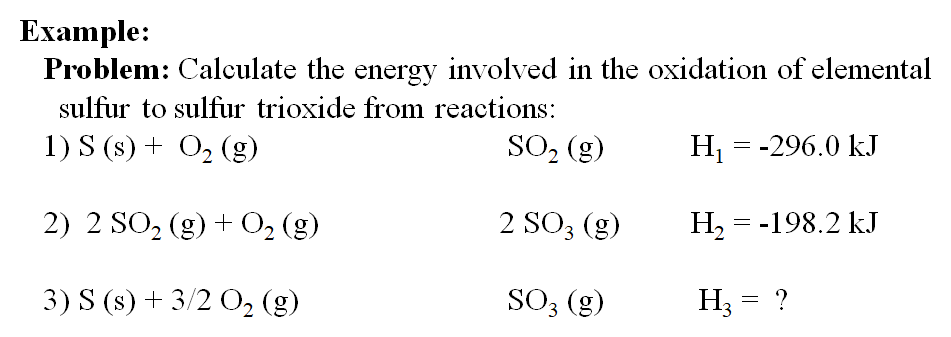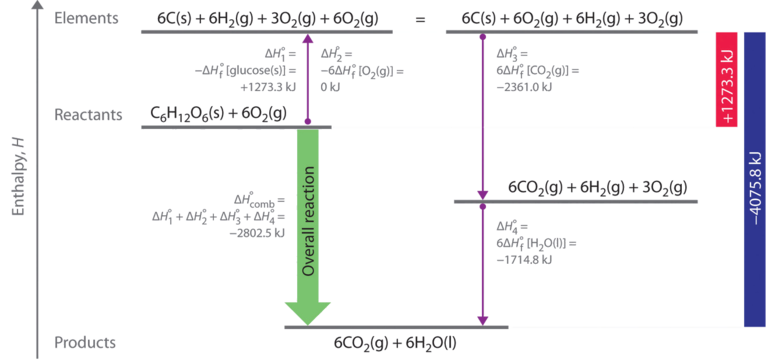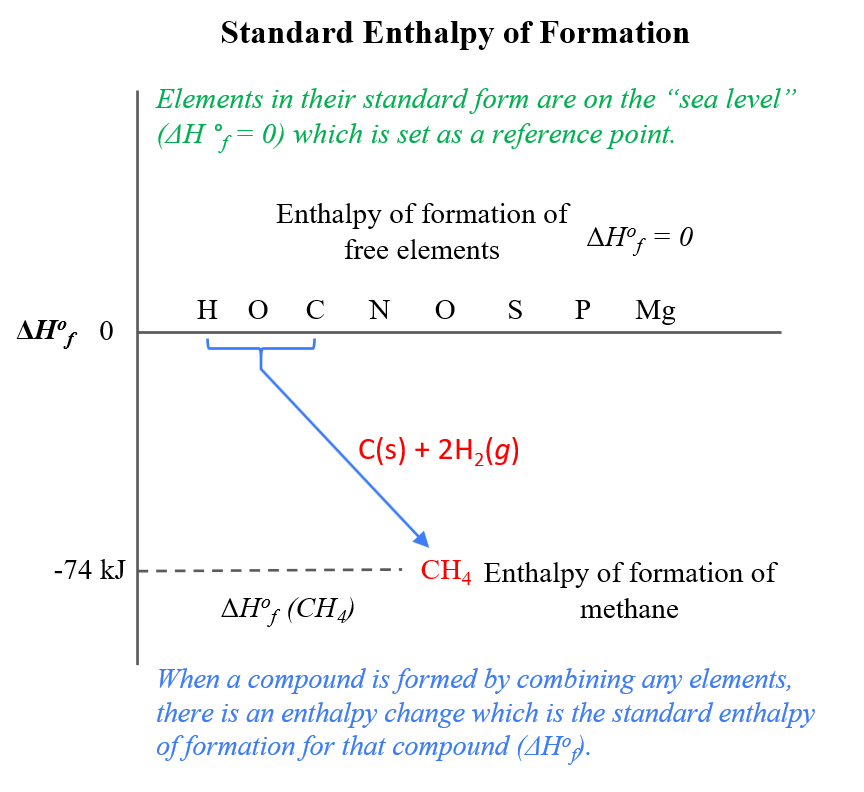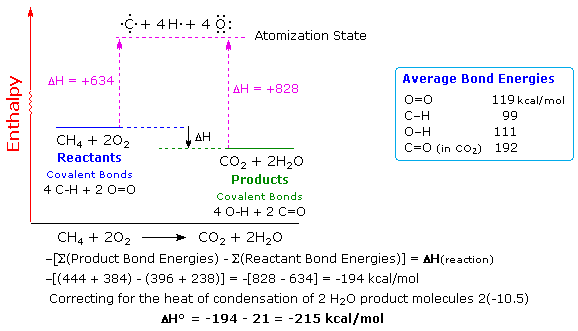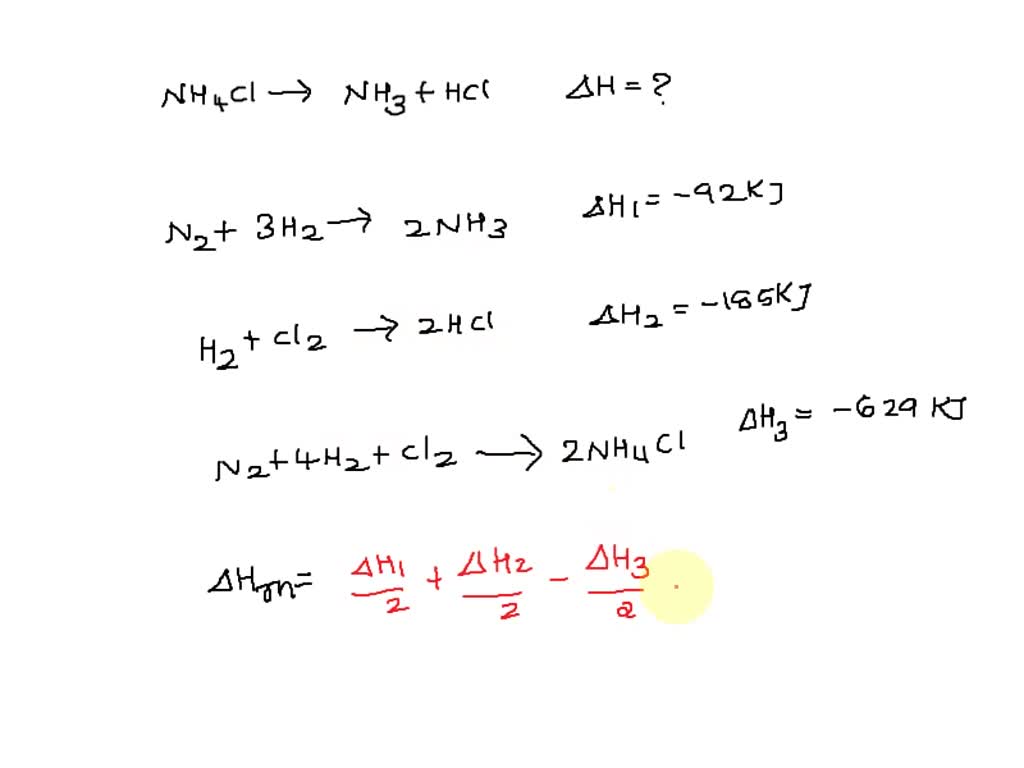
SOLVED: Given the following reactions, what is the overall enthalpy change for the following reaction? NH4Cl(s) —-> NH3(g) + HCl(g) ΔH = ? Use the following reactions to derive the ΔH of

Consider the following enthalpy diagram. What is the overall enthalpy change DHrxn for the - Brainly.com
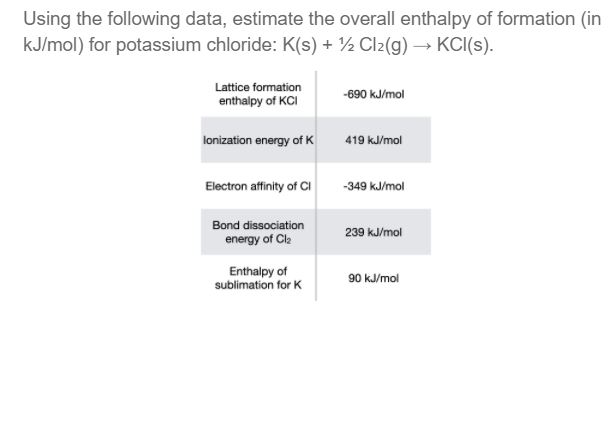
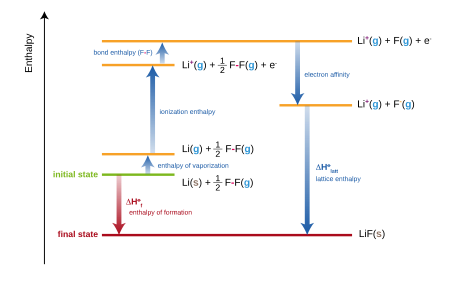
SOLVED: Using the following data, estimate the overall enthalpy of formation (in kJ / mol ) for potassium chloride: K(s)+1 / 2 Cl2( g) →KCl(s) . Lattice formation enthalpy of KCl -690